The world of chemistry seems challenging because there are many molecules with similar names in Katakana (the form of Japanese syllabic writing used especially for scientific terms, official documents, and words adopted from other languages). However, if you observe them as unique characters and visualize how they work, you may feel much closer to them. Here, we share some research results that were surprising: "It's like a living being!"
A research group at the University of Tokyo announced that by passing synthetic macromolecules through small holes in a material called a 'metal-organic framework (MOF),' they succeeded in identifying the arrangement (type and order) of monomers, which are the units of macromolecules. Depending on the type of monomer, some monomers 'spontaneously' entered the hole, much like a sea eel, while others did not, thereby identifying the sequence of the monomer. If the research is continued and established as a sequencing technology for reading out the monomer sequence of synthetic macromolecules, it will be useful for developing materials and may lead to the development of new information storage media in the future.

Provided by the University of Tokyo
Experiments inspired by biology
A macromolecule is a long, string-like giant molecule consisting of many monomers linked together. We are familiar with macromolecules, such as nylon, polyester, and polyethylene, as they are used in our daily lives, including clothing, containers, synthetic rubber, adhesives, and cosmetics. These are synthetic macromolecules created by humans. Natural macromolecules include starch, protein, DNA, RNA, natural rubber, and diamond.
Poly- is a prefix meaning 'many.' For example, the macromolecule 'polystyrene' is a molecule made up of multiple molecules of 'styrene' linked together. A 'polymer' is a substance that is an aggregate of macromolecules. However, some people use the terms 'macromolecule' and 'polymer' interchangeably.
Macromolecules are generally string-like, but are intertwined like spheres, making it difficult to read the monomer sequences. Meanwhile, inside the cells of living organisms, protein ribosomes trap elongated RNA in their tiny holes and read the monomeric sequence of bases or genetic information precisely. Inspired by these biological activities, the research group led by Associate Professor Nobuhiko Hosono, who is studying polymer chemistry at the School of Engineering at the University of Tokyo dreamed of sequencing the monomer sequences of synthetic macromolecules and set out to conduct experiments.
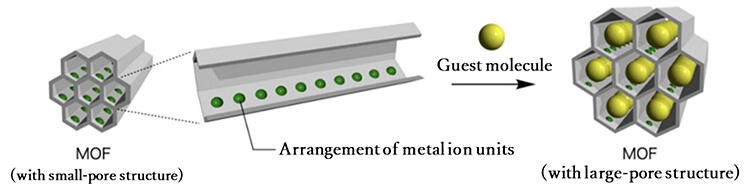
They focused on MOFs as a material with holes through which synthetic macromolecules could pass. MOFs consist of organic molecules bonded to metal ions. The lattice-like structure of MOFs are often compared to a jungle gym. MOFs are garnering attention as an excellent adsorbent because it can trap gases, such as carbon dioxide and methane, in the tiny holes in its skeleton. They were developed in 1997 by Distinguished Professor Susumu Kitagawa at Kyoto University.
Earthworms? Sea eel? "The unimaginable happens"
Considering the shape of the holes that would allow the macromolecules to easily pass through, Hosono and his colleagues chose MOF with 'honeycomb tunnel' type skeleton rather than a jungle gym type. The raw materials were ferrous ions and terephthalic acid, which is used in PET bottles. These ferrous ions are placed in a periodic pattern and cast on the walls of the tunnel-like hole. It is known that the lattice of this MOF changes as it absorbs molecules, and that the holes become larger. If this MOF accepts certain monomers into the hole and does not incorporate other ones, it can pave the way for sequencing.
Provided by the University of Tokyo
In the experiment, they first tried to incorporate polystyrene, which is used as a synthetic resin, into this MOF. However, the holes were not sufficiently large to accommodate it. Next, they tried a polymer of '4-vinylpyridine,' a monomer with the same structure and composition as polystyrene but with one carbon atom replaced by a nitrogen atom. The hole became larger and accepted the polymer. It was found that the ferrous ions in the holes and the nitrogen atoms of 4-vinylpyridine attract and combine well, causing the holes to enlarge. In other words, this MOF rejects styrene, accepts 4-vinylpyridine, and discriminates between the two.
So, how does a macromolecule get into a hole in the MOF? When Hosono was asked, "There is no way it crawls like an earthworm, right?" Hosono replied, "No, that's right, it crawls like an earthworm. It would be hard to imagine. But that it happens is the hottest point of this study. We often say they are sea eels because they go into holes. The author has not seen it in person, but the image is 'like a living creature.'"
The process is not very complicated: dissolve the macromolecules in a solution, add powdered MOF, and heat to 150 ℃. After a while, 4-vinylpyridine enters the hole. The ferrous ions seem to 'beckon' the 4-vinylpyridine to enter.
Here is a simple question. If the holes in the MOF are too small, the macromolecule cannot enter. Therefore, the nitrogen atoms cannot bind to the ferrous ions, and the holes will not become enlarged. If so, how would the first macromolecule get in?
Hosono said, "It is not yet known, like whether the chicken came first or the egg. It may be that only a few molecules bind at the end, and then a hole opens up."
Precise identification of the monomers and further findings...
Next, they tried a polymer of '2-vinylpyridine,' which looks exactly like 4-vinylpyridine, but the nitrogen atom is at a different position (structural isomer) and is located deeper in the molecular structure. The nitrogen atom was too far away from the ferrous ion on the wall of the hole to bind. Hence, 2-vinylpyridine did not enter the hole. They found that the position of the nitrogen atom in the monomer is key for the bonding and for the hole to be enlarged. Moreover, MOF identifies the monomer very precisely.

Provided by the University of Tokyo
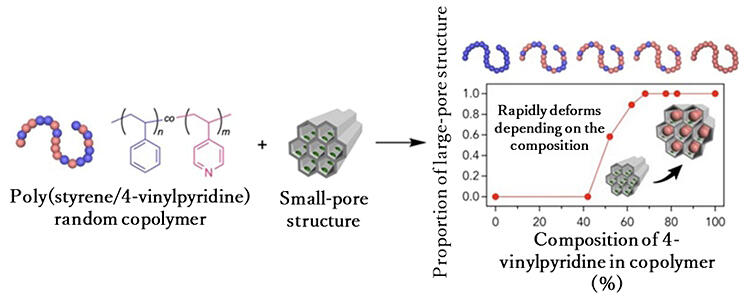
Further experiments were conducted. They used a 'copolymer' (macromolecules composed of several types of monomers) that was a mixture of styrene, which did not enter the hole in the previous experiment, and 4-vinylpyridine, which did. In the 'random copolymer' in which these monomers were irregularly connected, the copolymer did not enter the hole when the styrene content was high. However, when the 4-vinylpyridine content exceeded 50%, the holes suddenly became larger and allowed the copolymer to enter. Upon closer examination, it was found that this was due to the ferrous ions recognizing 4-vinylpyridine. "This is a great discovery that demonstrates the ability of the holes in the MoF to recognize monomer sequences in synthetic macromolecules." said Hosono.
Provided by the University of Tokyo
Copolymers include random and 'block copolymers,' which are two 'homopolymers' of a single type of monomer linked together. Until now, when the two were mixed, identifying and separating them was difficult.

Provided by the University of Tokyo
Random and block copolymers are prepared using equal amounts of styrene and 4-vinylpyridine. When MOF was added to these mixed solutions, only the block copolymers entered the hole. Even with only approximately 20% 4-vinylpyridine, the block copolymer entered the hole, but the random copolymer did not, a difference that allowed the two to be easily separated.
These results were published in the June 21 electronic edition of the international chemistry journal Chem and announced by the University of Tokyo on June 22.
Opens new ways for the application of macromolecules and MOFs
Although sequencing technology is available for DNA, a natural macromolecule, it is difficult for synthetic macromolecules. Hosono said, "Considering the fact that synthetic macromolecules can enter the holes in the MOF, it seems possible to do something new in the world of macromolecules. This achievement is a breakthrough that brings us closer to realizing monomer sequencing of synthetic macromolecules."
However, the current results are at the stage where they were able to identify the monomers whose monomer sequences were known in advance. When sequence information can be extracted from an unknown sequence, only then it can be called monomer sequencing. It is also necessary to advance research and respond to various monomers in the future. Once established, the technology could be used to develop, purify, and improve the functionality of polymeric materials. Hosono hopes that it will also help in the separation of plastics. It will also pave the way for new applications of MOF, which has been studied primarily as an adsorbent.
Hosono explained further, "If the array can be read precisely, it can be used as an information storage medium." Research on 'DNA storage' using DNA base sequences as a next-generation information storage technology is advancing worldwide, and this appears to be a synthetic macromolecular version of such storage technology. "DNA uses only four types of monomers, but synthetic macromolecules can use over 100 types, theoretically providing a tremendous amount of information capacity. I believe we can create an information innovation that will change our lives, perhaps in the distant future," he stresses.
Nylon and polyester, the raw materials for inexpensive clothing, were developed around 1940. Since then, plastics have replaced wood, metals, and glass, and synthetic polymers have become indispensable in our daily lives. In the future, humans may depend on polymers in ways that we cannot imagine today.
Journal Information
Publication: Chem
Title: Decoding polymer chains via gated inclusion into flexible nanoporous crystals
DOI: 10.1016/j.chempr.2023.05.041
(KUSAKA Takeo / Science Portal Editorial Office)
Original article was provided by the Science Portal and has been translated by Science Japan.




