Sulfur has attracted attention as a potential cathode active material for energy storage batteries, serving as a promising alternative to inorganic oxide materials. However, a significant hurdle in this pursuit has been the tendency of reaction intermediates to elute into the electrolyte during the charging and discharging processes, thus posing a longstanding challenge to enhance coulombic efficiency. In response to this issue, the Lithium-Sulfur Battery Team, under the leadership of Specially Appointed Professor Masayoshi Watanabe at the Institute of Advanced Sciences (IAS) in Yokohama National University (YNU), achieved a breakthrough. They have succeeded in suppressing the elution of these intermediates by developing a new-concept electrolyte, starting from the study on ionic liquids. The outcome of this innovation is a lithium-sulfur (Li-S) battery with high weight energy density.

Sulfur cathode with large theoretical capacity and low cost — Electrolyte elution of intermediates is a bottleneck
The demand for lithium-ion batteries (LIBs) is on the rise due to the widespread adoption of electric vehicles. However, ensuring a stable supply of battery materials continues to be a significant challenge. For instance, the cost of the rare metal cobalt has seen a substantial increase in recent years, raising concerns about the ability to maintain a steady supply. Sulfur has garnered significant attention because it is obtained from the desulfurization process of large quantities of imported crude oil and is also relatively abundant in volcanic Japan.
A single sulfur molecule has a ring structure comprising eight atoms, with 16 electrons participating in the charge and discharge processes. Consequently, the theoretical capacity reaches 1672 mAhg-1, approximately ten times greater than that of a typical lithium-ion battery (LIB) cathode. The Li-S battery harnesses this advantage by employing sulfur (S) as the positive electrode and lithium (Li) as the negative electrode (Fig. 1).
Fig. 1: Schematic of a Li-S battery
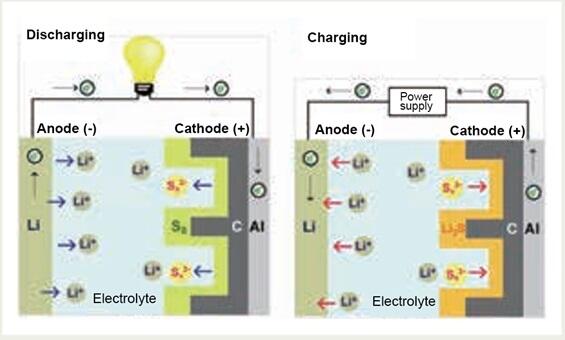
Nonetheless, numerous challenges persist, including issues such as short circuits stemming from inadequate lithium dissolution and deposition reactions. Additionally, sulfur expands by as much as 80 % in volume during the discharging process. "Among other issues, lithium polysulfides (Li2S𝓍), intermediates formed during the charge-discharge reaction of Li-S batteries, elutes into the electrolyte, resulting in a significant increase in discharge capacity and reduced coulombic efficiency, commonly referred to as the shuttle effect." explained Watanabe.
Practical large pouch cell prototype achieves high gravimetric energy density
Watanabe and his colleagues tackled this issue through a two-pronged approach. Firstly, they focused on developing electrolytes in which the reaction intermediates would not dissolve. Their attention turned to ionic liquids; a field of study Watanabe had been involved with for some time. Ionic liquids are often referred to as a 'third liquid,' following water and organic solvents, because they consist solely of ions. They are non-combustible, exhibit low volatility, and are efficient ionic conductors. However, they are characterized by high viscosity, and thus far, none of them have met the necessary transport properties essential for battery applications.
As a solution to this challenge, Watanabe and his team devised a 'solvate ionic liquid' by mixing equimolar quantities of Li salts with 'glyme,' a non-protic polar molecule known for its strong affinity to lithium ions. Watanabe emphasizes several critical factors they took into account during this process. They believed that the relatively favorable transport properties and the decreased solubility of ionic materials would mitigate the dissolution of Li2S𝓍. Additionally, from a safety perspective, he highlights the significant advantage of using ionic liquids due to their lower flammability.
This marked a significant breakthrough, leading to the subsequent development of sulfolane electrolytes with improved performance. The team prototyped a large-scale cell that combined this electrolyte with a titanium black-added cathode and bis(fluorosulfonyl)amide (FSA) anion-added electrolyte. This configuration yielded an impressive gravimetric energy density of 350 Wh kg-1 (Fig. 2).
Fig. 2: Li-S pouch cell

The other approach is from the cathode. As sulfur is an insulator, a composite mixed with conductive carbon is employed for the positive electrode. However, only microporous carbon is utilized, and sulfur is trapped within the pores to prevent elution. Challenges persist, including a reduced energy density stemming from excessive carbon usage, but the outlook is optimistic.
These advancements have sparked a wide array of research endeavors. Watanabe commented, "In the future, we aim to address issues such as cycle life and rate capability, which determine how quickly the battery can charge and discharge. We will also actively pursue the development of Li-S polymer batteries utilizing polymers and batteries featuring lithium sulfide (Li2S) as the positive electrode."
A battery with a Li2S cathode has already undergone testing to assess the performance of the cathode material. Furthermore, collaborative research with the Consortium for Lithium-Ion Battery Technology and Evaluation Center (LIBTEC), a technical research association, is currently in progress. Li-S batteries have the potential to emerge as the next generation of energy storage solutions, garnering worldwide attention.
(Article: Kazuyuki Katayanagi, Photography: Hideki Ishihara)




