A research group of the University of Hyogo has announced that it has succeeded in stabilizing ammonia, which is normally a gas at room temperature, in a solid state. This was achieved by encapsulating it in a boric acid aggregate. This achievement has the potential to dramatically ease the handling of ammonia (a hazardous substance), which is attracting attention as a means of storing hydrogen, which is a next-generation energy source. This result was not what was expected from the beginning, and the researcher who discovered it said, "I was amazed at the wonder of nature."
Ammonia: A substance with high expectations but gaseous and difficult to handle
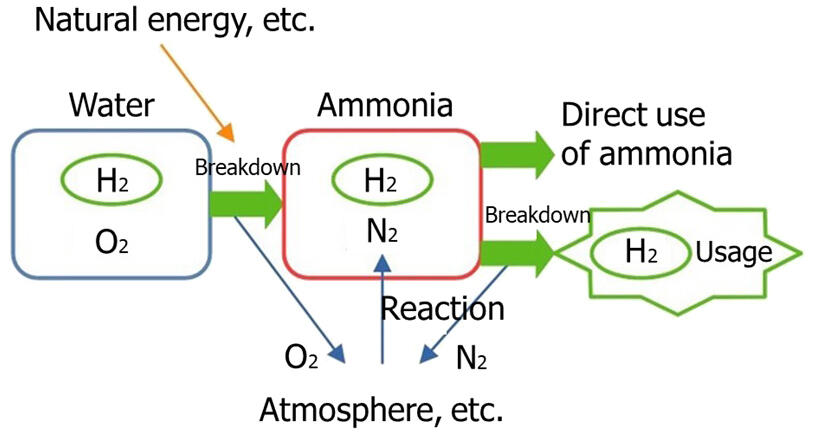
While ammonia has long been used as a raw material for fertilizers and chemical products, it has been showing increasing promise in the energy field recently. Since the generation of electricity from natural energy sources such as solar and wind is affected by weather and time of day, electricity storage technology is needed that allows electricity to be retrieved when needed. Therefore, a promising method is to use the electricity generated to break down water to produce hydrogen, which can then be stored. The method of reacting hydrogen and nitrogen, such as in the atmosphere, to produce ammonia for storage has been focused. This process of producing ammonia is also presented in high school chemistry textbooks as the "Haber-Bosch process."

Provided by the University of Hyogo
Ammonia has a melting point of −78℃ and a boiling point of 33℃. It is a colorless, transparent gas at room temperature. Further, it has a strong pungent odor and is toxic. Hence, it is designated as a hazardous substance. It becomes liquid at about 8 atmospheric pressure and is stored in cylinders. To promote its widespread use, safe and more efficient storage and transportation methods need to be developed to make it easier to handle.
Recently, news reports mentioned that if you wrap ammonia in something, you can make it solid even though it is at room temperature and society will be able to enjoy it successfully. The author, who is not familiar with chemistry, was intrigued by this news, thought it sounded like "deep fried ice cream," and contacted Professor Emeritus Masao Morishita, who studies chemical thermodynamics, at the University of Hyogo, who led this research effort. Mr. Morishita retired from the university in March of this year. However, the results of this research made him realize that he was "unlikely to stop his research." He has been working as a special researcher at the National Institute for Materials Science since April.
An experiment contemplated for 5 years, and now it was time to perform it!
Initially, the author asked: "If ammonia can be made solid at room temperature, it can be made small. Even though it is a hazardous substance, it can be handled with ease. So did you perform the experiment aiming for such a breakthrough?" Morishita remained silent for a moment and said: "Hmmm. In retrospect, I would say that's certainly a summary of what happened. Because... actually, I could not fully express it in the paper or the press release, but the research was originally aimed at something else entirely."
The research was initially aimed at making "ammonia borane (an ammonia-boron compound)," a type of substance that stores hydrogen, at a low cost. He continued saying, "Ammonia borane is quite expensive, but I had been warming to the idea for about five years that 'we could make it cheaper this way.'" I decided to give it a try after two students chose topics for their master's and graduation theses. To be specific, the experiment was aimed at making ammonia borane by dissolving boron oxide in an aqueous ammonia solution, followed by freezing it in liquid nitrogen at −196 ℃ and then removing the water (in the form of ice)."
An "impossible result," the opposite of disappointment
Morishita said, "I will never forget May 23 of last year." The students came to Morishita with the data from the X-ray analysis of the products. Looking disappointed, they said, "Sorry Sir! We couldn't make ammonia borane."
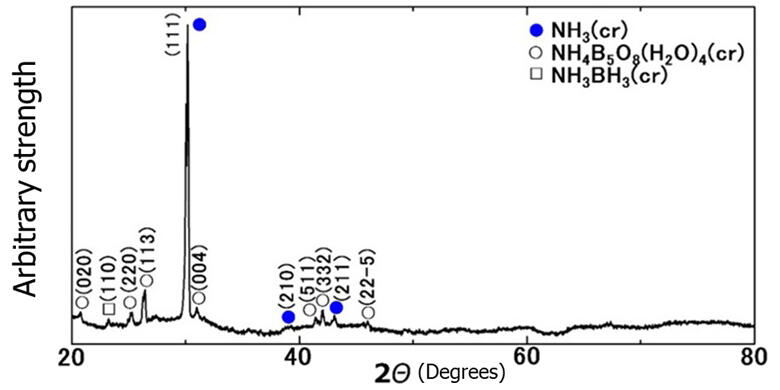
Though he told them, "It can't be helped, let's change the topic of the paper," he instructed the students to bring the analysis results of other molecules formed alongside. Mr. Morishita's eyes were caught by the surprising data that showed the conspicuous presence of solid ammonia, even though it was at room temperature. "That's impossible," he said. The solids were distinctive in size and cubic in shape and certainly crystals of ammonia. In aqueous solutions, ammonia changed to ammonium ions and boron oxide changed to boric acid ions. When water was removed from the solution, it was inferred that ammonia was encapsulated by the aggregates of boric acid.
Provided by the University of Hyogo
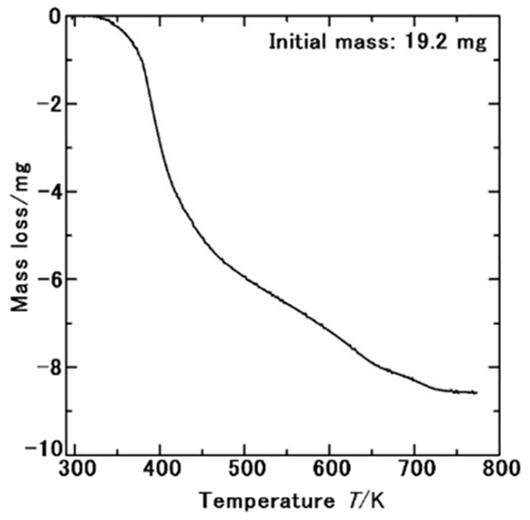
When heated, ammonia remained as a solid till 52℃, even above the room temperature. Normally, the melting point of ammonia is −78℃, which means that ammonia remained as a solid for 130℃ above its melting point. Theoretical calculations confirmed that this ammonia type remained as a solid at room temperature. "To use an analogy, it's something like 'ice in boiling water will melt, but it won't melt if you wrap it in a plastic paper.' I felt there was no way this could really happen. However, it happened and was the world's first successful achievement of ammonia solids above room temperature," said Morishita. He further said that a small amount of ammonia borane, which was originally intended for preparation, was also produced. The weight of the ammonia solids was determined.
Provided by the University of Hyogo
Ammonia, a hazardous substance, is difficult to handle in universities and laboratories unless it is made into an aqueous solution. "We cannot allow students to handle gases and liquids. However, in this experiment, there was no smell of ammonia at all. In other words, it remained in a solid form, and there was no sense of uneasiness at all," said Morishita.
Use in Energy: The potential for a major transformation
If ammonia can be handled as a solid at room temperature, it would be a much safer substance to humans. Consequently, its potential for use as a hydrogen storage material will also expand. "Until now, people have not had the idea of using solid ammonia as an industrial material. If it can be put to practical use, it may lead to revolution in energy use," said Morishita.
The aforementioned results were published on May 27 in RSC Advances, a journal of the Royal Society of Chemistry (UK), and announced by the University of Hyogo on May 25.
The issue for the future, above all, will be to clarify the mechanism by which ammonia remains solid at room temperature. There must be some energy at work here. If it is "deep fried ice cream," which the author thought of before the interview, the ice cream in the batter stays cold. However, ammonia produced in this research was fundamentally different in that it was solid even though it was at room temperature, which seems to be a mystery.
Provided by the University of Hyogo
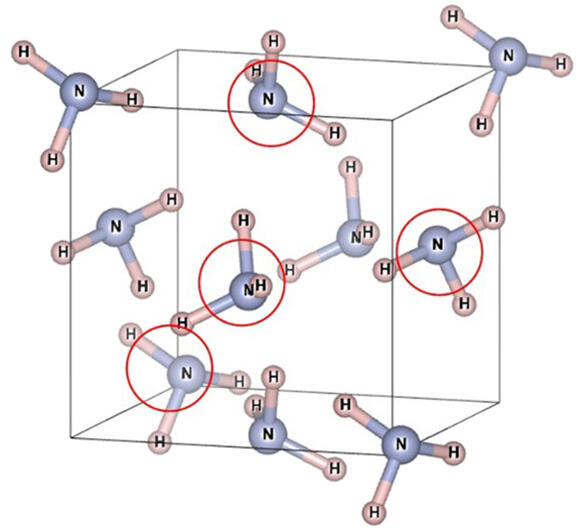
The "B-N bond" between boron (B), which makes up boric acid, and nitrogen (N), which makes up ammonia, is a strong and well-known bond that may be involved in this mystery. It is likely that this bonding or the pressure generated in the ammonia bonds by this bonding is at play.
To extract hydrogen from ammonia, it must be heated to over 400℃; however, Morishita is also researching ways to do it at lower temperatures. In the experiment, liquid nitrogen at −196℃ was used to transform ammonia into a solid; however, he says there is room to investigate ways to do this at higher temperatures.
The appeal of science is that you don't understand it until you try it!
"This is a promising achievement for the practical application of hydrogen energy. But what I found most interesting was the fact that ammonia could maintain a solid state up till 130℃. This surprised me and made me experience the mysteries of nature. Ammonia is an interesting substance for basic science and abundant in the universe. In the solar system, for example, it is found in the interiors of Uranus and Neptune. It is also related to amino acids, which are essential for the birth of life," said Morishita.
This achievement seems to be a product of "serendipity," meaning that a person who continues to work hard accidentally discovers something of value other than the intended result. In the history of science and technology, the discoveries of X-rays and penicillin are representative examples of serendipity. Research is a slow and laborious process, and it does not always develop according to a schedule for anticipated outcomes.

(screenshot taken during the online interview.)
Morishita said: "It was a great pleasure for me as a researcher to encounter miraculous physical phenomena that are beyond human knowledge, and I was excited together with my students. Perhaps it is because I have been conducting basic thermodynamic research for more than 30 years that I did not miss this phenomenon." The interview reconfirmed the value and appeal of science, which can only be understood by trials.
(KUSAKA Takeo / Science Portal Editorial Office)
Original article was provided by the Science Portal and has been translated by Science Japan.




