A research group of the University of Tokyo and collaborators has discovered that the addition of a liquid glue component to the enantiomer of a boron compound (compound used for cancer radiotherapy but seen to be practically not applicable as a drug) improved its accumulation and retention in cancer cells to levels exceeding those of drugs used in clinical practice. Boron neutron capture therapy (BNCT) using this method almost completely eradicated cancer in mice. Moving forward, the research group aims to apply the method to human pancreatic cancer and other cancers that are difficult to cure completely.
Provided by Associate Professor Takahiro Nomoto of the University of Tokyo
BNCT, a form of radiotherapy, kills cancer cells by radiation and other means produced within the range of about a single cell when low-energy thermal neutrons collide with boron atoms. In 2020, BNCT was covered by insurance for some unresectable head and neck cancers. Currently, L-BPA is used as a boron agent because it is efficiently taken up by cancer cells.
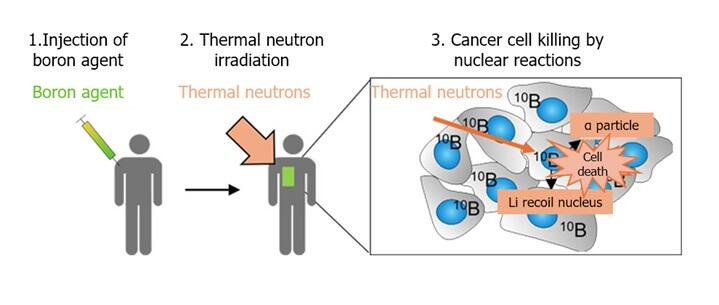
After boronophenylalanine (BPA) is injected, patients undergo irradiation with thermal neutrons produced by accelerators to kill BPA-containing cells.
Provided by Associate Professor Takahiro Nomoto of the University of Tokyo
L-BPA is taken up by cancer cells through cancer cell-specific transporters up to the clinically required concentration and kills cancer cells upon thermal neutron irradiation. However, there are also challenges. For example, intratumor L-BPA is gradually cleared via the transporters over time after uptake, and L-BPA is also taken up and discharged by normal cells through different transporters.
Associate Professor Takahiro Nomoto at the Graduate School of Arts and Sciences of the University of Tokyo, who studies drug delivery systems, which are techniques to deliver drugs to specific sites in the body, wondered, "Is there a way to deliver the boron agent more selectively to cancer cells and keep it in the cells for a longer period." In 2020, he discovered that the addition of poly(vinyl alcohol) (PVA), which is used in liquid glue, to L-BPA facilitates L-BPA uptake by cancer cells through vesicles formed by membrane invagination. He thought that D-BPA, the L-BPA enantiomer (one of a pair of molecules of which structures are mirror images of each other, like right and left hands), which is not taken up efficiently by cancer cells and has not been considered useful for cancer therapy, might become useful if used together with PVA, and decided to test this idea.
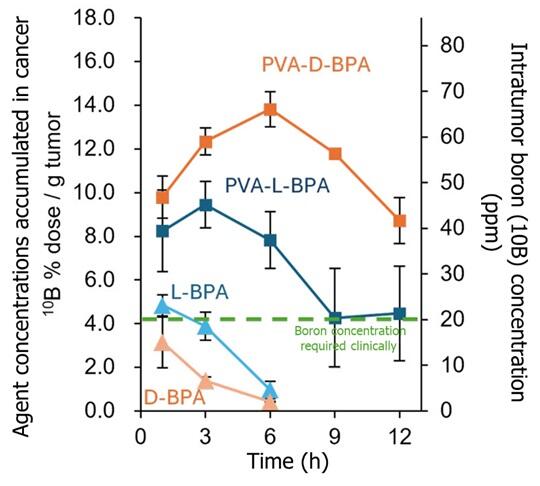
In cultured cells derived from mouse colon cancer and human pancreatic cancer, D-BPA mixed with PVA was efficiently taken up by the cancer cells through vesicles, as was the case for L-BPA. Moreover, animal experiments on the intratumor accumulation and retention levels over time in mice showed that D-BPA remained at higher intratumor concentrations for longer periods of time than L-BPA. According to Nomoto, the likely reason is that intracellular D-BPA is not discharged through transporters, while L-BPA is.
Provided by Associate Professor Takahiro Nomoto of the University of Tokyo
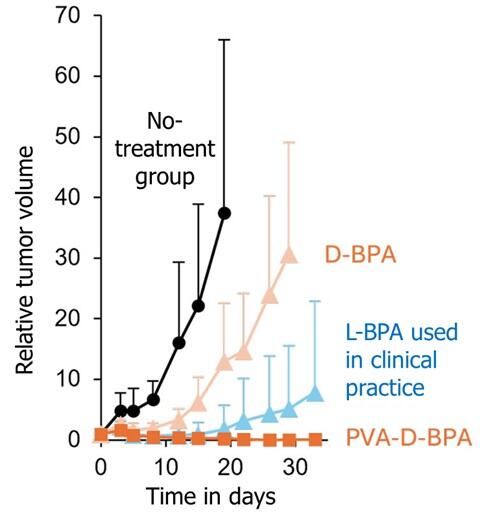
When the effectiveness of BNCT in actual cancer treatment was tested in mice, D-BPA mixed with PVA was so effective that it reduced the volume of cancer more than L-BPA (without PVA), which is actually used in clinical practice and achieved a radical cure.
Provided by Associate Professor Takahiro Nomoto of the University of Tokyo
Nomoto said, "D-BPA mixed with PVA is expected to be effective even for cancers that did not adequately respond to conventional drugs."
Moving forward, the research group hopes to standardize the agent and conduct animal experiments before applying it to humans.
This study was conducted jointly by Kyoto University and Stella Pharma (Chuo City, Osaka Prefecture) and was supported by the Japan Science and Technology Agency (JST), the Japan Agency for Medical Research and Development (AMED), Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, and Joint research fund from Stella Pharma. The findings were published in the electronic edition of the Journal of Controlled Release, a journal specializing in drug delivery systems, on December 3, 2024.
Original article was provided by the Science Portal and has been translated by Science Japan.




